LARYNGECTOMY.NET
Phase III: Randomisation
The third and last phase of the PETAL project is the comparative phase. It represents on its own a multicentre and prospective interventional study.
The primary objective of this phase is to evaluate the capacity of the PETAL programme to improve the quality of life of laryngectomised patients, while distributing in 12 French and Belgian oncology centres.
The design of the study is based on the collective randomisation model, or on a cluster randomisation trial. In this type of trial, the subjects included in the study are not randomised individually, instead it is groups of subjects that are randomised. A group of subjects is called a cluster and in this case corresponds to the patients of a specific hospital. In practice, this experimental design is widely used to assess the efficacy of patient education programmes (HAS, 2007a, 2007b).
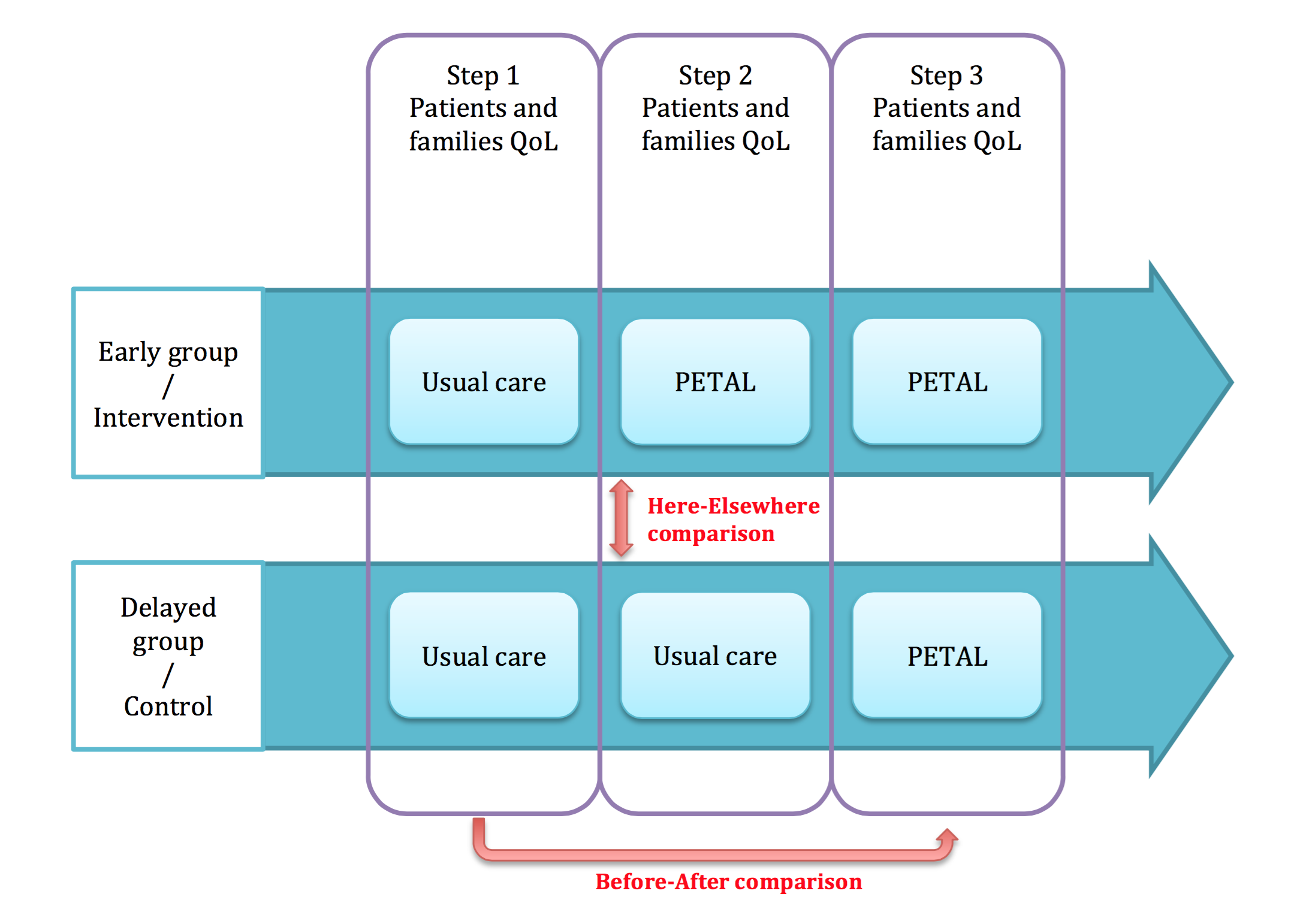
Thus, each of the 12 centres will be randomised as case (PETAL programme) or control (education usually practised in the centre). Therefore, there will be 6 centres or clusters in the intervention arm (PETAL programme), and 6 clusters in the control arm. This will allow an initial here-there type comparison of quality of life scores between case clusters and control clusters.
In order to avoid that the randomised centres in the control refuse in the end to participate in the study, the PETAL programme will be installed in these centres at the end of the study. Thus, the control arm will also be called the late arm, while the case arm will also be called the early arm. The quality of life scores will be compiled in each centre before and after setting up the PETAL programme. This will allow a second before-after comparison of quality of life scores between patients included before surgery, and those afterwards.
The primary endpoint is the score of the Performance Status Scale for Head and Neck cancer patients (PSS-HN) that assesses the quality of life (QOL) in patients with UADT cancer. The H0 hypothesis tested is that the PETAL programme will improve the PSS-HN score by 10 points.
The calculation of the number of subjects required is based on the comparison of the means of the total score of the PSS-HN scale at 6 months post-laryngectomy taking into account the cluster randomisation (the patients from a single cluster are not independent), formulating the following working hypotheses:
– An expected improvement of 10 points in the mean scores of the intervention group patients (PETAL programme) relative to the control group during the here-there comparison period.
– Bilateral test and common variances in both groups (standard deviation of 20 points)
– alpha risk of 5%
– power of 80%
– intra-class coefficient of correlation of 0.05;
– a number of clusters of 6 per randomisation arm.
The estimated number of subjects required is 22 subjects per cluster (centre), i.e. 132 patients per arm for a total of 264 patients included in the study.
Phase III schematic diagram: